| There are no translations available.
Une pléthore de publications sur sport et santé montre l’intérêt pour les séropositifs plus que pour les personnes séronégatives à pratiquer une activité sportive. Il est etabli que l’activité physique améliore les parametres liés à la fatigue, au poids et à l’indice de masse corporelle. Par contre , il ne semblerait pas qu’il y ait un effet sur les dyspneés, et sur les parameters immunitaires(CD4 et Charge virale) (Smith, Barbara A et alAIDS: 13 April 2001 – Volume 15 – Issue 6 – pp 693-701). Cette dernière constatation semble devoir être remise en cause par les travaux de Micolena Veljkovic et Nevena Veljkovic qui démontrent la stimulation par l’exercice physique de la production d’anticorps anti VIP(peptide vasoactif intestinal , possédant un large spectre d’action) et que le peptide VIP possède un mimétisme moléculaire avec une partie hautement conservée de la proteine virale gp120, mais aussi avec des marqueurs tumoraux du cancer du sein , de l’uterus et de la prostate. D’autre part le taux d’autoanticorps est corrélé négativement à la progression de la maladie. Enfin les LTP(long term progressors ) et LTS(long term survivors) possèdent un taux élevé de ces auto anticorps. Ainsi s’ouvre une voie nouvelle alternative aux antiviraux avec leur cortège d’effets indésirables et de résistance. Des essais thérapeutiques contrôlés restent à faire pour mesurer l’effet de l’activité physique sur les CD4 et la charge virale sur des patients naîfs d’abord, puis sur d’autres déjà traités en vue d’allèger ou de supprimer les antiviraux. Seuls les pouvoirs publics sont à même de financer ce type d’essai, les firmes pharmaceutiques ayant tout à perdre de tels essais!
THE ROLE OF EXERCISE IN PREVENTING AND TREATING HIV INFECTION AND CANCER Milena Veljkovic1, Nevena Veljkovic2, Violeta Dopsaj3 1 Laboratory for Radioisotopes, Institute of Nuclear Sciences Vinca, P.O.Box 522, 10001 Belgrade, Serbia Abstract It is shown in this article that physical exercise can significantly contribute to prevention and suppression of disease development in HIV infected individuals. Findings presented in this review show that physical exercise induces production of anti-vasoactive intestinal peptide (anti-VIP) antibodies in the sera of both HIV infected and non-infected individuals by increasing the level of VIP protein. Negative correlation observed between progression of HIV disease and level of anti-VIP antibodies titer in HIV infected patients reflects decrease in amount of HIV particles present in the circulation, which occur as a consequence of the anti-VIP antibodies’ high affinity for HIV gp120 antigen. Taking into account deficiencies of currant medical therapies for AIDS and lack of prospective vaccine, aerobic physical exercise can be considered as highly promising and widely accessible non-toxic therapy for AIDS.
Introduction At the beginning of the third millennium humans are faced with a HIV/AIDS pandemic with probabilities that has never been seen before. The most effective way to control this pandemic would be development of safe and effective HIV vaccine. Unfortunately, despite the commitment of enormous scientific and financial resources in the past 15 years, no vaccine candidate is on the immediate horizon. In addition, current medical therapy of HIV disease is extremely toxic (including multiple side effects and drug interactions), expensive, and constantly under the risk of emerging drug-resistant HIV strains. Clearly, other less toxic and inexpensive treatments must be pursued to slow down the spread of HIV and decrease the burden of disease as well as the burden of treatment. In this article the exercise will be proposed as an important contribution to the solution for these vexing problems. It has been shown that the exercise promotes the development of antibodies (Anti-VIP/NTM) in both normal and HIV individuals, with a specific affinity (or cross reactivity) to the HIV-1 envelope protein (gp120 surface antigen) of HIV. As such, HIV particles are bound in the circulation and removed before reaching their target cell (CD4), thereby reducing the risk of systemic infection with HIV, which would occur after exposure (disease transmission). Evidence in the literature also supports the idea HIV disease progression can be slowed down and damaged immune system can be recovered by the increase titer of this unique antibody (either by passive immunization or by exercise). Using exercise as a front line therapy in HIV could be considered as a « natural vaccine » whose wide application could contribute to a worldwide slowing of HIV spread. Here we also describe mechanism of interaction between anti- vasoactive intestinal peptides (VIP) autoantibodies and tumor marker, which is highly homologous with HIV-1 envelope protein expressed on > 90% breast, gynecological and prostate tumor cells and point out exercise as useful supportive cancer therapy.
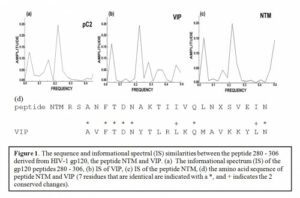
Useful Antibodies in the HIV disease If an Achilles’ heel exists in HIV, it might be in the central portion of gp120 (residues 280-302, RSANFTDNACTIIVQLNESVEIN, designated as peptide NTM [20], Figure 1d). This portion of the molecule is highly conserved in all known HIV variants and appears to be crucial for viral infectivity. In fact, researchers have demonstrated that a minimal change in this sensitive site completely abolishes HIV infectivity. Unfortunately, this part of gp120 is not immunogenic in humans [47] because the immune system treats this part of gp120 as « self », likely due to similarity of peptide NTM and several human proteins [48]. However, antibodies found in both HIV+ and seronegative individuals recognize this region of the HIV-1 gp120 molecule (residues 280 – 302). Additionally, Neurath and coworkers showed that these antibodies are significantly more prevalent in asymptomatic HIV positive individuals, compared to AIDS patients [7].When passively administered the NTM Abs can efficiently delay HIV disease progression [3]. Therefore, it seems that NTM recognizing antibodies are involved in control of HIV infection. Upon investigating the possible origin of these antibodies, they appear to represent autoantibodies (antibodies which are not generated by foreign antigens) against certain human antigen. In order to identify candidates for this autoantigen, human sequences from Swiss-Prot protein database were analyzed using the Informational Spectrum Method. This method is used for the analyses of protein sequences by signal processing techniques. According to this approach, protein sequences are transformed into signals by assignment of numerical values to each amino acid. These values correspond to the electron-ion interaction potential [9], determining electronic properties of amino acids responsible for their intermolecular interactions [10]. The signal obtained is then decomposed in periodical functions by Fourier transformation. The result is a series of frequencies and their amplitudes. The obtained frequencies correspond to the distribution of structural motifs with defined physico-chemical characteristics responsible for the biological function of the protein. The informational spectrum calculated for the peptide 280-306 is presented in Figure 3a. As can be seen, it contains only one characteristic pick corresponding to the frequency F (0.218). The computer assisted search of the Swiss-Prot database revealed the vasoactive intestinal peptide (VIP) as the only protein among analyzed human proteins that possesses such a spectral property (Figure 3b). It is important to note that the second dominant common frequency component F (0.031) in the informational spectra of VIP and the peptide NTM, has been previously identified as a carrier of the spectral characteristic that represent information which is responsible for the interaction between HIV and the CD4 receptor [49].
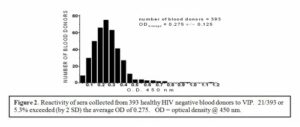
Therefore, it has been proposed that antibodies identified by Neurath and coworkers represent natural anti-VIP autoantibodies. In one of the recent reports sera collected from 393 HIV– negative blood donors were screened by ELISA, based on the peptide NTM. Results of this analysis demonstrated that approximately 5% (21/393) of the sera tested contain significant titer of the anti-VIP/NTM antibodies corresponding to > OD average value (ODaverage) + 2 SD.
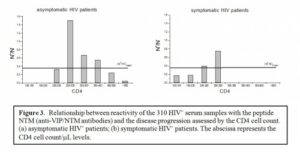
For HIV+ individuals, the amount of anti-VIP/NTM antibodies available appears to strongly correlate with progression of HIV disease. Results of current studies (see Figure 3) demonstrate that the level of anti-VIP/NTM reactive antibodies in HIV patients in the first stage of illness (CD4 > 500/ml), when the immune system efficiently controls HIV, is very low, and similar to levels in normal HIV– people (Figure 3a). However, the amount of these antibodies significantly increases in disease stages, corresponding to CD4 values between 200 and 500/ml. Below that CD4 level (<200) the anti-VIP/NTM antibody levels sharply decrease (Figure 3b). In the terminal stages of disease the amount of NTM-reactive antibodies in sera of HIV+ patients appears to be significantly decreased.
A unique method to produce high titers of VIP/NTM reactive antibodies may be available to both HIV– and HIV+ individuals. An interesting article by Paul and Said from 1988 [16] showed that autoantibodies to VIP were present in plasma from 29.6% of healthy (non-HIV+) human subjects who habitually performed aerobic muscular exercise (running, cycling, swimming, aerobic dancing, and/or weight training, 3 or more workouts per week for a year or more prior to study entry), compared to 2.3% of healthy subjects who did not perform regular exercise. The antigenic stimulus for the formation of these autoantibodies could not be identified from their data, however, acute exercise has been shown to be associated with a brisk increase in plasma levels of VIP [50,18]. It is certainly plausible to theorize that these antibodies may have been produced in response to increased VIP levels during exercise.
VIP and immune system VIP is a 28-amino-acid pleiotropic peptide with broad biological actions including potent vasodilatator and brochodilatator activity. It is also neurotransmitter and immunomodulator which plays an important role in immune homeostasis. VIP acts as modulator of immune function, modulating the mobility and adherence of lymphocytes and macrophages, phagocytic cell functions (phagocytosis and free radical production), the lymphocyte proliferative response, lymphokine and immunoglobulin production and the NK cell activity [43]. VIP participates in the intricate cytokine network by controlling local immune responses through down-regulation of IL-2 and IL-10 mRNA expression in T-cells and by significant reduction of the stability of newly synthesized IL-4 protein [33]. It also modulates the inflammatory functions through down-regulation of the expression of proinflammatory cytokines as TNF-a, IL-12, IL-1, IL-6 and NO (nitric oxide). Existence of different receptors for prepro-VIP-derived peptides on lymphocytes, suggests that VIP may be considered as important immunoregulatory molecule [22]. VIP stimulates IL-2 release at low concentrations with a marked effect at 10(-14) M that gradually returns to control levels by 10 (-7) M [5]. On the other hand, it can also inhibit the production of IL-2 by either unfractionated spleen cells, or by purified CD4+ T cells in a dose-dependent manner. This inhibition is specific, since structurally related peptides, such as secretin and glucagon, have little or no inhibitory effect. VIP induces a rapid increase in intracellular cAMP in CD4+ T cells. Investigations of this phenomenon by northern blots showed that VIP downregulated IL-2 mRNA, indicating the possibility of a transcriptional regulatory event [23]. This conclusion is supported by another study which showed that VIP inhibits IL-2 and IL-4 production through different molecular mechanisms. IL-2 production was regulated at the transcriptional level through the downregulation of IL-2 mRNA, whereas the production of IL-4 was modulated at the posttranscriptional level [28]. Through its downregulatory effect on IL-2 and IL-4 production, locally released VIP could potentially affect T-cell development within the thymus [13]. The CD4 and CD8 spleen cells represent at least two of the cellular targets for VIP inhibition of proliferation [15]. VIP also up-regulates the costimulatory activity of macrophages for Ag-primed CD4+ T cells.
VIP and physical activity Several studies demonstrated increase in plasma VIP concentration as reaction to physical exercise. Marked increases in peripheral plasma concentration of VIP [1.8 (rest) vs. 22.3 pmol/l (3 h)] during 3h period of mild bicycle exercise were reported [12,32]. Another study of 24 military cadets went through a five days period of heavy physical exercise (35% of VO2 max) demonstrated that plasma concentration of VIP increased two- to five-fold during the physical training, with the highest increase on the day two[40]. Similar study performed during a ranger training course lasting for five days with almost continues physical activity, demonstrated increase of the plasma VIP concentration from 8.8 pmol/l to a maximum of 23.4 pmol/l on the second day of the course [41].The same researchers have investigated effect of exercise in twelve subjects participating in a 90-km cross-country ski race lasting 4.45 – 6.50 h. Plasma concentration of VIP was greatly increased immediately after the race, and the level was not normalized within 140 minutes, though there was a significant decrease after 80 minutes of rest [42].Oektedalen and co-workers have reported that VIP increased 2-fold in twenty young men participated in a five day training course with prolonged and heavy physical exercise [52]. In seven subjects with exercise-induced asthma, plasma VIP rose significantly 5 min after the test, in comparison with the controls [36]. Study of healthy subjects performing ergometer exercise with progressive increases in workload until exhaustion, lasting from 16 to 32 minutes, and with a corresponding maximum energy output of 1500 to 5100 W, showed an increase in plasma VIP concentration from a pre-stimulatory level of 3.3 pmol/l to 5.6 pmol/l [53]. Opstad reported that plasma VIP increased during physical exercise lasting from more than 20 minutes with a workload of more than 50% of VO2 max [4]. According to this study, further increase took place in the early recovery period to a maximum level 5 – 10 minutes after the exercise. Significant increase in plasma concentration of VIP during exercise was also demonstrated by Paul and co-workers [24]. Plasma concentrations of VIP was analyzed in six male endurance runners and six male hockey players before, during and 15 min after 90 min treadmill running at 65% maximum oxygen uptake [26]. Plasma VIP level increased significantly beyond resting levels for both groups (endurance runners, 76.1 ng/l; hockey players 155.6 ng/l). This pleiotropic peptide must be under strong control. Autoantibodies directed against VIP are potent modifiers of its biological actions [27] and muscular exercise may influence the incidence of these antibodies.
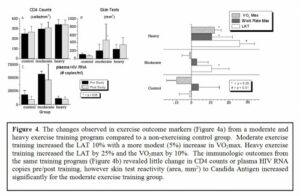
The potential role of aerobic exercise training in treating and preventing HIV infection The human immunodeficiency virus type 1 (HIV-1) carries on its surface a protein designated as « gp120 ».It has been demonstrated that antibodies with specific affinity to gp120 surface antigen (residues 280-302, designated peptide NTM), representing autoantibodies (designated as anti-VIP/NTM antibodies) with a specificity to VIP [20,21], are involved in control of the HIV disease progression [31,38]. Recently, aerobic exercise training, stimulating formation of anti-VIP/NTM antibodies [16] was proposed as a way to increase the anti-VIP/NTM antibodies titer in both normal and HIV+ individuals [38]. Increased titers of anti-VIP/NTM antibodies induced by exercise in HIV negative individuals is important because the increased titers of anti-VIP/NTM antibodies could bind HIV particles in the circulation and remove them prior to reaching their target cell, thereby reducing the risk of systemic infection with HIV, which would occur after exposure. Therefore, aerobic exercise may be an important, inexpensive, non-toxic, widely available front line therapy in HIV, acting as an immune stimulant (for both HIV+ and HIV– individuals) and representing a type of “natural vaccine” that could contribute to a worldwide slowing of HIV spread. During the first step of HIV infection gp120 binds to the receptors on the cell surface of the host, allowing the HIV virus to enter the cell. The central portion of this gp120 molecule has an immunoglobulin-like structure which facilitates participation in the immune network. Therefore, immediately after infection HIV tries to fit the host idiotype by producing thousands of variants of gp120. This process of adaptation usually takes years, and during this time the host immune system is more or less able efficiently to control HIV disease. In some HIV-infected persons this period is short, and in others it can be quite long, resulting in « slow » or « fast » disease progression. After this latent period, a separate fraction of viruses, whose gp120 carries the host idiotype, will be established [17,44,45]. This population of HIV becomes accepted by the host immune system as « self » and therefore protected from the host’s immune attack. Even worse, these gp120 molecules will be included in regulation of the immune network, destabilizing its vital components and accelerating progression of disease [46]. In this way HIV may escape from the latent period and progressively destroy the immune system of the infected person. Several prior studies on aerobic exercise training in HIV+ individuals have demonstrated that it is safe, effective, and has a number of beneficial outcomes [1,6,8,9,10,14,19,34]. The aerobic exercise fitness improvements include a 10-25% improvement in lactic acidosis threshold (LAT) and 5-10% increase in maximal oxygen uptake (VO2max) depending on the exercise training intensity (see Figure 4a). In addition, despite concerns about the stress of aerobic exercise on already damaged immune systems (specifically increases in infections, morbidity, or mortality), there have been no documented adverse effects of aerobic exercise training in HIV+ patients at either moderate or heavy exercise training levels [19]. The available literature clearly supports the premise that aerobic exercise is well tolerated in HIV+ individuals. In regard to immunologic improvement through aerobic exercise training, there are no evidence that CD4 counts or viral loads substantially improve during the exercise intervention [19], although it was shown that reactivity to Candida antigen improve with moderate exercise (Figure 4b). Finally, it has been demonstrated, that quality of life improves significantly with aerobic exercise training, in comparison with non-exercising control group.
VIP/NTM-reactive autoantibodies and cancer Recently, Rakowicz-Szulczynska and co-workers have reported that 95% of breast and gynecological cancer cases in women and prostate cancer cases in man express on the cell surface the HIV-1 gp120-like tumor antigen, which is 90% homologous with HIV-1 Env protein [35,37,39,51,54]. Also, strong reactivity of this tumor marker with the monoclonal neutralizing antibody elicited by V3 loop has been demonstrated [2]. Authors hypothesized that this tumor antigen represents the Env protein of some human endogenous retrovirus which is evolutionary close to HIV-1. Rakowicz-Szulczynska have defined procedure for detection and therapy of breast and gynecological cancer, which is based on application of the anti-HIV-1 Dupon’s commercial monoclonal antibody [25]. It is of interest to speculate about possible role of the HIV-1 Env-like protein expressed on the surface of a malignant cell. HIV-1 Env protein resembles several important features of human immunoglobulins and might encodes a host idiotopes [11]. Idiotope-bearing Env, either soluble or exposed in multiple form on the surface of the cell, can influence the immune system in iditype(Id)-anti-Id fashion [29]. It means that tumor cells which expose on their surface HIV-1 Env-like marker, encoding the host idiotype, will be protected from attack of the immune system. This mechanism of protection could be especially important for metastatic tumor cells which are in circulation and are directly exposed to attack of the immune system. Like HIV and the HIV-infected cells, cancer cells, which express the HIV-1 Env-like tumor marker could be a target for the VIP/NTM-reactive autoantibodies that can eliminate these cells from circulation. For this reason, these antibodies potentially represent an important factor in control of cancer development, which can slow-down disease progression in cancer patient. It should be noted that effect of exercise, which is based on activity of the VIP/NTM-reactive autoantibodies, will be prolonged in comparison with other above described short-term effects. Potential Impact Aerobic exercise training has been shown to be a very promising, non-toxic, non-drug adjunctive therapy that improves aerobic fitness, increase quality of life, and potentially improve immune status (Candida skin test reactivity) in HIV+ individuals. If the future study provides evidence that aerobic exercise training can increase the titer of anti-VIP/NTM antibodies in normal individuals (potential to decrease the risk of HIV transmission) and in HIV+ individuals (potential to slow disease progression), it would be a widely available intervention which is both non-toxic and has no drug interactions. This type of intervention to slow disease progression in HIV+ individuals or decrease the risk of systemic HIV infection after exposure in HIV– Individuals would certainly be widely available and applicable in both developed and developing countries. References Bradac JA, Mathieson BJ. An epitope map of immunity to HIVHIV-1: a roadmap for vaccine development. Div of AIDS, NIAID, NIH 1991
|


Laisser un commentaire